Pharmaceutical engineering - Training
Mission :
Referencing and content promoting of biopharma training from profit and non profit organisations specialised in pharmaceutical engineering.
Training centers :
All training organisations are selected to be recognised and already partners of pharmaceutical & vaccine industries. This is the value of their experience and their team we want to promote on this site.
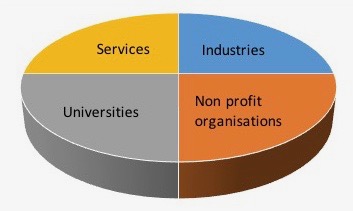
Industries :
Industries producing technologies for pharmaceutical industry invest a lot of efforts in R&D (process, instrumentation…). To communicate these new technologies (Opportunities and use) they have developed specific training program to their customers or prospect.
Services :
Industry of services have developed specific skills (Engineering office, Validation, Maintenance...). Their expertise cross different input in project management, regulation, normes, technologies... to satisfy their customers. These practices generates specific methodologies and knowledge they can share internaly and sometimes they develop specific training program externaly. This is a way to promote their knowledge and expertise we want promote on this site.
Non profit organisations :
Non profit organisations are the place where all actors can cross their expertise around a speciality (Engineering, process, clean & sterile, medical device, regulation normes, good practices…). It is the place where industries (Techno/services), regulation, pharmaceutical company, experts can have open discussion, share knowledge, methods and have a good overview of the state of the art.
Universities :
Universities open its knowledge through a dedicated short training program for industries and professionals. Associated R&D laboratories are often involved in these training program to propose and promote their high level of expertise and technology.
Subjects :
All selected training course covers technologies used by pharmaceutical laboratories in a context of production. (Including regulatory cGMP, ICH... and normes ISO... standards that are applicable in the pharmaceutical and vaccine industry)



Subjects are from :
- Bioprocess equipment
- Regulation (ICH, cGMP...)
- Normes (ISO)
- Validation
- Qualification
- Testing strategy
- BioPharma maintenance
- Clinical trials
- Medical device
Training details :
We attach importance about information detailing trainings. Clear objectives and program, valorisation of speakers, valorisation of the training center and all didactic methods, tools or equipment used to perform the training.
- Detailed programs
- Experimented speakers
- Details on location, price and date

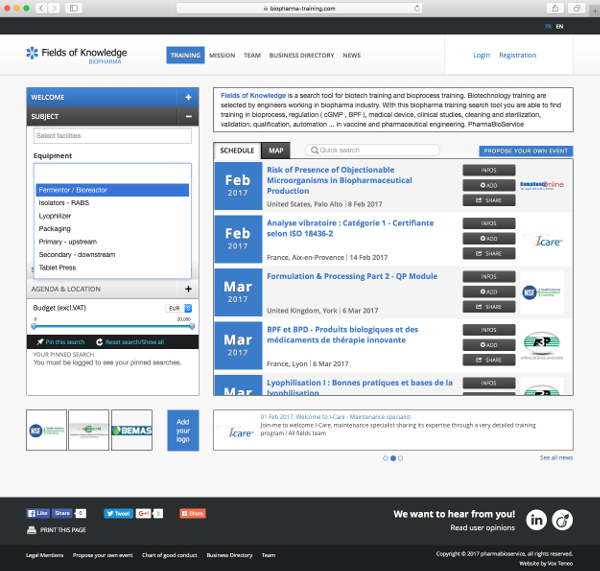
A structured training search tool dedicated to biopharma industry :
The search tool is dedicated to pharmaceutical and biological industry. This to help you finding a subject, organisations, location, date or budget constraints.
- Select your budget
- Use the free search tool
- Select trainings from one organisation
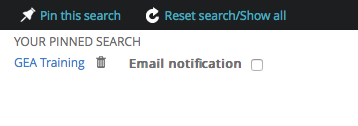
Notification :
You can set up a notification. Select and track all event on your defined criteria.
- Price
- Training provider
- Subject
- Location
- ...
Team - Values
- We serve our partners to the benefit of the sector and professionals
- We cultivate an atmosphere of trust
- We select training based on their quality
- Our approach is loyal and responsible
- We communicate openly and in a constructive manner
- We learn from mistakes
- We are modest
- We relay diversity
- We are friendly to each other
- We behave ethically
Contact :
mail : training@pharmabioservice.com